Percent Yield of Chemical Reactions Calculator
Introduction
Calculating the percent yield of chemical reactions is crucial for chemists to assess the efficiency of a reaction. A reliable tool for this purpose is a Percent Yield Calculator. This article provides a working HTML and JS code for the calculator, along with essential information on its usage, formula, examples, FAQs, and a conclusion.
How to Use
- Enter the actual yield in grams in the designated input field.
- Input the theoretical yield in grams in the respective space.
- Click the “Calculate” button to obtain the percent yield.
Formula
The percent yield is determined using the following formula:
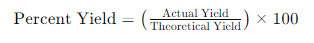
Example
Suppose you conducted a chemical reaction, and the actual yield is 25 grams while the theoretical yield is 30 grams. The percent yield is calculated as follows:
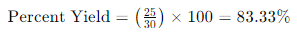
FAQs
Q1: What is the significance of calculating percent yield?
A1: Percent yield reveals the efficiency of a chemical reaction, providing insights into how much product is obtained compared to the theoretically predicted amount.
Q2: Can the percent yield be greater than 100%?
A2: In some cases, experimental conditions may lead to a percent yield exceeding 100%, indicating a higher-than-expected actual yield.
Q3: Is there a specific unit for percent yield?
A3: Percent yield is expressed as a percentage (%), representing the ratio of the actual yield to the theoretical yield multiplied by 100.
Conclusion
The Percent Yield Calculator simplifies the assessment of chemical reaction efficiency. By utilizing the provided HTML and JS code, chemists can effortlessly determine the percent yield, aiding in the optimization of experimental conditions and resource utilization.
